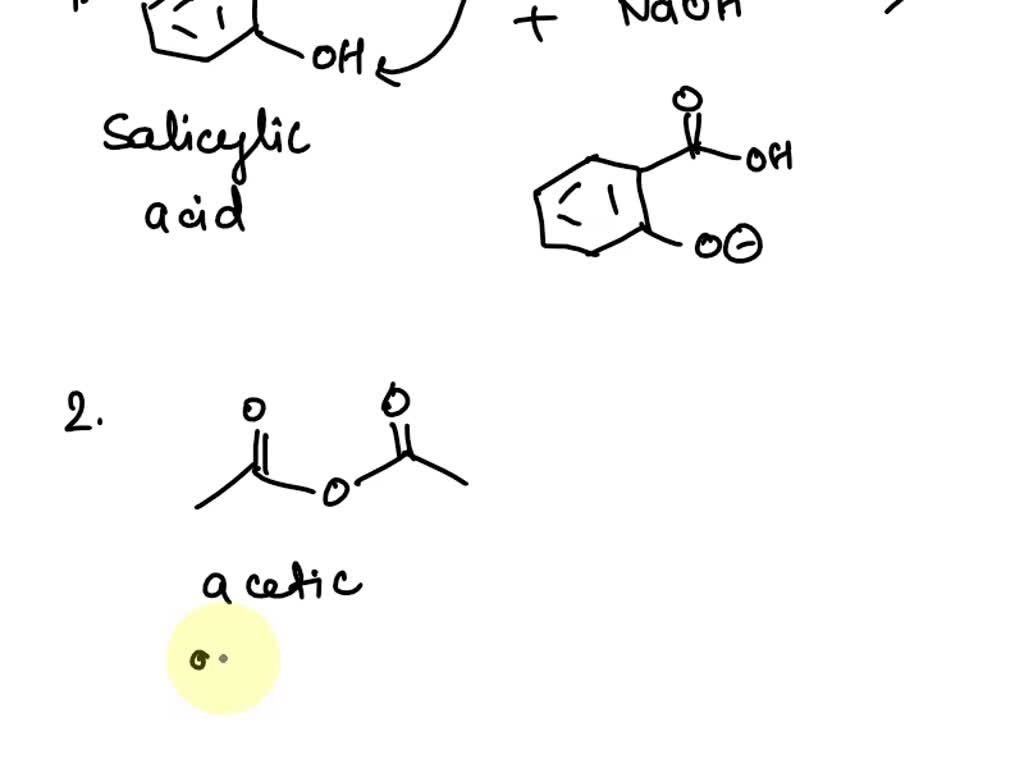
Aspirin Chemical Reaction . Why is the aspirin washed with cold water? Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. Aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. The molecular weight of aspirin is 180.16g/mol. It uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives acetylsalicylic acid (aspirin). Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: According to the data in the merck index, if 1.0 g of aspirin is dissolved in 100 ml of water at 37. Conduct a chemical reaction to produce aspirin.
from www.numerade.com
Why is the aspirin washed with cold water? The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. Conduct a chemical reaction to produce aspirin. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. It uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives acetylsalicylic acid (aspirin). The molecular weight of aspirin is 180.16g/mol. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: According to the data in the merck index, if 1.0 g of aspirin is dissolved in 100 ml of water at 37. Aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride.
SOLVED Draw a curly arrow mechanism for the synthesis of aspirin from
Aspirin Chemical Reaction Conduct a chemical reaction to produce aspirin. Conduct a chemical reaction to produce aspirin. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. Why is the aspirin washed with cold water? The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. According to the data in the merck index, if 1.0 g of aspirin is dissolved in 100 ml of water at 37. The molecular weight of aspirin is 180.16g/mol. It uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives acetylsalicylic acid (aspirin). Aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride.
From favpng.com
Aspirin Chemical Synthesis Chemical Reaction Pyridine Design, PNG Aspirin Chemical Reaction Conduct a chemical reaction to produce aspirin. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. It uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives acetylsalicylic acid. Aspirin Chemical Reaction.
From studylib.net
EXPERIMENT 3.8 ASPIRIN SYNTHESIS Aspirin Chemical Reaction According to the data in the merck index, if 1.0 g of aspirin is dissolved in 100 ml of water at 37. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: It uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives acetylsalicylic acid (aspirin). Why. Aspirin Chemical Reaction.
From www.freepik.com
Premium Vector Aspirin chemistry chemical formula structure vector Aspirin Chemical Reaction Aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. According to the data in the merck index, if 1.0 g of aspirin is dissolved in 100 ml of water at. Aspirin Chemical Reaction.
From www.chegg.com
Solved Give the reaction mechanism for the synthesis of Aspirin Chemical Reaction Why is the aspirin washed with cold water? The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. Conduct a chemical reaction to produce aspirin. According to the data in the merck. Aspirin Chemical Reaction.
From studylib.net
aspirin synthesis & analysis Aspirin Chemical Reaction The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. Conduct a chemical reaction to produce aspirin. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. It uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3. Aspirin Chemical Reaction.
From www.researchgate.net
Paracetamol and aspirin pathway. Side reactions solvation of aspirin Aspirin Chemical Reaction The molecular weight of aspirin is 180.16g/mol. According to the data in the merck index, if 1.0 g of aspirin is dissolved in 100 ml of water at 37. Conduct a chemical reaction to produce aspirin. Aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Why is. Aspirin Chemical Reaction.
From chemisfast.blogspot.com
Aspirinuse of aspirin and aspirin from phenol . PG.CHEMEASY Aspirin Chemical Reaction Why is the aspirin washed with cold water? The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. Conduct a chemical reaction to produce aspirin. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. Aspirin is prepared by chemical synthesis from. Aspirin Chemical Reaction.
From mavink.com
Aspirin Molecule Structure Aspirin Chemical Reaction Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Why is the aspirin washed with cold water? Aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride. Conduct a chemical reaction to produce aspirin. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h. Aspirin Chemical Reaction.
From ibalchemy.com
D.2 Aspirin and penicillin IB Alchemy Aspirin Chemical Reaction The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. According to the data in the merck index, if 1.0 g of aspirin is dissolved in 100 ml of water at 37. Conduct a chemical reaction to produce aspirin. Aspirin is prepared by chemical synthesis. Aspirin Chemical Reaction.
From www.alamy.com
Chemical Structure of Aspirin, Anatomy Of Aspirin, Molecular structure Aspirin Chemical Reaction Conduct a chemical reaction to produce aspirin. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. Why is the aspirin washed with cold water? Synthesis of aspirin (acetylsalicylic acid) reaction scheme. Aspirin Chemical Reaction.
From www.slideserve.com
PPT Aspirin PowerPoint Presentation, free download ID5345611 Aspirin Chemical Reaction Why is the aspirin washed with cold water? Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride. It uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4),. Aspirin Chemical Reaction.
From www.saubhaya.com
Chemical Makeup Of Aspirin Saubhaya Makeup Aspirin Chemical Reaction Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Why is the aspirin washed with cold water? It uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride gives acetylsalicylic acid (aspirin). According to the data in the merck index, if 1.0 g of aspirin is dissolved. Aspirin Chemical Reaction.
From studylib.net
Experiment 7 Synthesis of Aspirin Aspirin Chemical Reaction Aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. According to the data in the merck index,. Aspirin Chemical Reaction.
From mavink.com
Struktur Kimia Aspirin Aspirin Chemical Reaction Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. Conduct a chemical reaction to produce aspirin. The molecular weight of aspirin is 180.16g/mol. Why is the aspirin washed with cold water? It uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4), where the salicylic acid treated with acetic anhydride. Aspirin Chemical Reaction.
From webapi.bu.edu
The melting point of aspirin. The melting point of aspirin 128. 2022 Aspirin Chemical Reaction Conduct a chemical reaction to produce aspirin. Aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. The molecular weight of aspirin is 180.16g/mol. According to the data in the merck. Aspirin Chemical Reaction.
From chemisfast.blogspot.com
Aspirinuse of aspirin and aspirin from phenol . PG.CHEMEASY Aspirin Chemical Reaction Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride. The molecular weight of aspirin is 180.16g/mol. According to the data in the merck index, if 1.0 g of aspirin is dissolved in 100 ml of water at 37. It uses a reaction of esterification catalyzed acid (h. Aspirin Chemical Reaction.
From www.coursehero.com
[Solved] please draw an organic chemistry reaction of synthesis of Aspirin Chemical Reaction Aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Conduct a chemical reaction to produce aspirin. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is. Aspirin Chemical Reaction.
From www.reddit.com
Do these reactions make sense? Asked to show chemical equations for Aspirin Chemical Reaction According to the data in the merck index, if 1.0 g of aspirin is dissolved in 100 ml of water at 37. Conduct a chemical reaction to produce aspirin. Aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride. Why is the aspirin washed with cold water? The molecular formula for acetylsalicylic acid is c 9. Aspirin Chemical Reaction.